Title: Children Oxygen Administration Strategies Trial-Nutrition.
- Duration: 50 months
- Start Date: August 2018
- End Date: October 2022
Background:
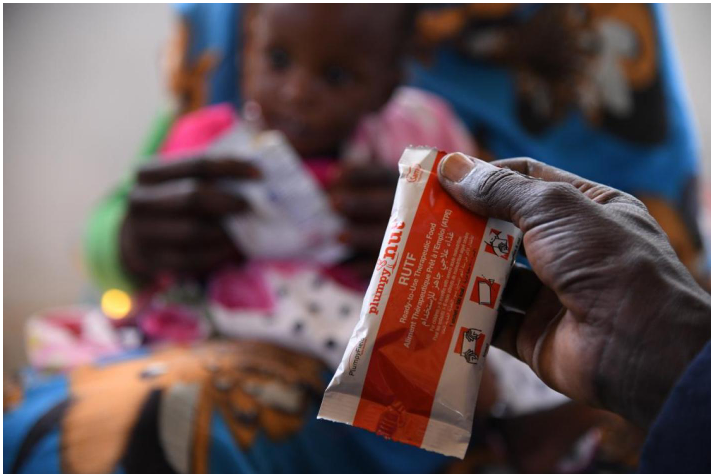
To prevent poor long-term outcomes (deaths and readmissions) for children who are hospitalised with severe pneumonia the integrated global action recommends the importance of continued feeding but gives no specific recommendations for nutritional support. Early nutritional support is practiced in a wide variety of critically ill patients in order to provide vital cell substrates, antioxidants, vitamins, and minerals essential for normal cell function and decreasing hypermetabolism. COAST-Nutrition overarching objective is to investigate whether supplementary feeding for 56-days (8 weeks) using Ready to Use Therapeutic Feeds (RUTF) in addition to usual diet in children aged 6 months or more and without severe acute malnutrition versus usual standard care (usual diet alone) will improve outcome at 90-days and 180 days to provide a better evidence base for future management guidelines.
The study is an open, multicentre RCT of 840 participants, aged from 6 months to 12 years, admitted to hospital with respiratory distress complicated by hypoxia. Participants will be enrolled over a 36-months period and followed up to 28 days post-randomisation, Day 90 and day 180 (6 months) to assess short and long-term outcomes following admission with pneumonia. Ancillary (sub) studies include molecular characterization of bacterial and viral pathogens, evaluation of putative biomarkers of pneumonia, assessment of muscle and fat mass and host genetic studies.
Children will be enrolled at admission to hospital from 5 sites in 2 countries (Uganda and Kenya) and followed for 180 days. The trial has a pragmatic design, to ensure this encompasses a spectrum of high-risk children, identified largely by clinical criteria, so that the results are applicable to health services in Africa, with limited access to health technologies.
Aims and purpose
Primary objectives
To establish whether supplementing feeds with RUTF will improve anthropometric outcomes at Day 90 and Day 180.
Secondary objectives
The key secondary outcome measures are:
- Survival to 28 days and 180 days (6 months)
- Disability-free survival to 28 days
- Re-admission to hospital by 28 and day 180 days
- Neurocognitive sequelae at 90 days
- Anthropometric status by 28 days, Day 90 and Day 180 (MUAC, TST and WHZ)
Expected benefits to the society
The trial will investigate and provide evidence to whether nutritional support after severe illness does improve outcome this will provide important new evidence that is both clinically beneficial and important in improving healthcare. This study is the first step in providing an option for nutritional support following severe pneumonia and will help in the design of a large Phase III trial.
Registration: ISRCTN10829073 (6th June 2018) PACTR202106635355751 (2nd June 2021)
Funding:
- Joint Global Health Trials scheme: Medical Research Council Department for International Development Wellcome Trust (MR/L004364/1))
- European and Developing Countries Clinical Trials Partnership (EDCTP) (RIA2016S-1636)
Investigators:
Prof Kathryn Maitland, Dr Mainga Hamaluba (Kilifi, Kenya) Prof Sarah Kiguli, Dr Robert Opoka, Dr Shela Oyella, Prof Peter Olupot-Olupot, Dr Florence Alaroker, Dr Abner Tagoola (Uganda) Dr David Harrison, Prof Kathy Rowan (ICNARC) Prof Thomas Williams, Prof Andrew Bush (Imperial College, London)
Clinical Queries
In the first instance, all clinical queries should be passed to Prof. Kathryn Maitland (k.maitland@imperial.ac.uk)
And
All lay public queries to the COAST_Nutrition Administrator at KEMRI Wellcome Trust Programme
Phyles Maitha; PMaitha@kemri-wellcome.org Tel: +254715461761
Dissemination of results
The results will be disseminated in a virtual meeting to be held on the
28th March at 0800HRS GMT
Investigators’ meeting
A physical investigators’ meeting will be held in Kilifi in May 2023

Funding Partners


Consortium Members



 Deutsch
Deutsch